Practice Free A00-282 Exam Online Questions
Define.xml is an XML-based submission of a clinical study’s:
- A . metadata
- B . protocol
- C . results
- D . data
What is the definition for treatment-emergent adverse event (TEAE)?
- A . AE that started or worsened after the first dose of study drug
- B . AE that continued after the first dose of study drug
- C . AE that started 6 months after the last dose of study drug
- D . AE that improved after the first dose of study drug
The following SAS program is submitted:
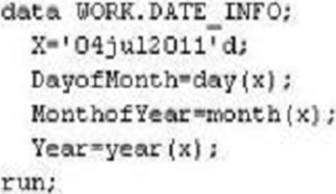
Which types of variables are DayofMonth, MonthofYear, and Year?
- A . DayofMonth, Year, and MonthofYear are character.
- B . DayofMonth, Year, and MonthofYear are numeric.
- C . DayofMonth and Year are numeric. MonthofYear is character
- D . DayofMonth, Year, and MonthofYear are date values
Identify the data structure with the following characteristics:
– Contains one or more records per subject, per analysis parameter, and per analysis timepoint.
– May be derived from findings, events, interventions and special-purpose SDTM domains, or other ADaM datasets.
– A record can represent an observed, derived, or imputed value required for analysis.
General Data Structure (GDS)
Basic Data Structure (BDS)
Subject Level Analysis Data Set (ADSL)
Event Level Analysis Data Set (ADAE)
You are working on validating the ADSL data set which contains the variables subject, sex, age, birth date, baseline height, and baseline weight. These variables are brought in from SDTM, and you derive new variables baseline BMI (from baseline height and weight) and age categorization (from age).

You get the following in the SAS log:
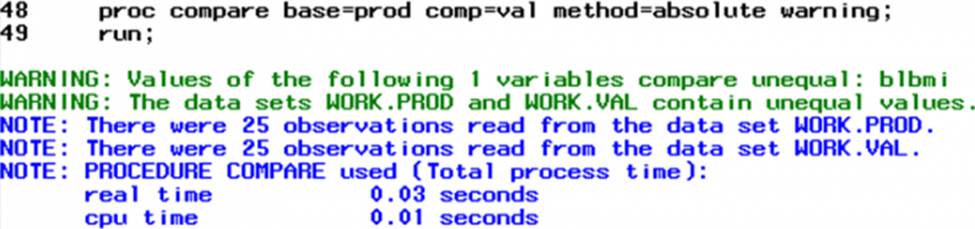
To reconcile this difference, which variable values will be most helpful to provide to the production programmer?
- A . BLBMI
- B . SUBJID BLBMI
- C . AGECAT1 BLBMI
- D . SUBJID BLHT BLWT BLBMI
What is the primary purpose for specifying identifier variables across SDTM domains?
- A . To uniquely reference each record
- B . To satisfy regulatory conditions
- C . To include the appropriate subject information
- D . To include the appropriate study information
Given the following code executed with system date and time of April 29, 2020 at 10:33 AM:
data _null_;
daytim=put("&sysdate"d,date9.)||" "||put("&systime"t,time8.);
call symputx("nowdate", daytim);
run;
%put &nowdate;
The output of the macro variable "nowdate" in the log will be:
- A . &nowdate
- B . 29APR2020 10:33 AM
- C . daytim
- D . 29APR2020 10:33:00
Identify the CDISC model with the following characteristics:
XML-based content and format standard facilitates the archive and interchange of the metadata and data for clinical research provides an accurate audit trail that is 21 CRF Part II compliant
- A . Trial Design Model (TDM)
- B . Study Data Tabulation Model (SDTM)
- C . Operational Data Model (ODM)
- D . Analysis Data Model (ADaM)
An action plan that describes what will be done in a drug study, how it will be conducted, and why each part of the study is necessary is called:
- A . a clinical trial plan
- B . a protocol
- C . a data management plan
- D . a statistical analysis plan
Given the full data set SUBJECT.DEMO:

The following SAS program is submitted:
%macro MP_ONE(pname=report);
proc &pname data=SUBJECT.DEMO;
run;
%mend;
%MP_ONE(print)
%MP_ONE()
Which PROC step(s), if any, execute?
- A . PROC PRINT only
- B . PROC REPORT only
- C . PROC REPORT and PROC PRINT
- D . No PROC steps execute
